| Catalog No. | KMF104022 |
|---|---|
| CAS No. | 2747918-21-2 |
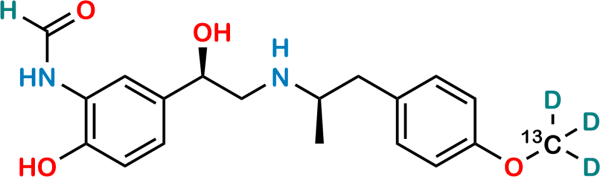
| Formula | C19H21N2O4D3 |
| FW | 348.4 |
| Unit | Price | Stock |
|---|---|---|
| 100 mg | Enquire | Enquire |
| Purity | >95% |
| Storage | Store at 2-8 deg. C for long term storage |
| Shipping | Product is stable to be Shipped at Room Temperature |

| Catalog No. | KMF104022 |
|---|---|
| CAS No. | 2747918-21-2 |
| Formula | C19H21N2O4D3 |
| FW | 348.4 |
| Unit | Price | Stock |
|---|---|---|
| 100 mg | Enquire | Enquire |
| Purity | >95% |
| Storage | Store at 2-8 deg. C for long term storage |
| Shipping | Product is stable to be Shipped at Room Temperature |
CAS No. : 183814-30-4
CAT No. : KMF104044
Formula : 2(C19H24N2O4) : C4H4O4 : 2(H2O)
FW : 2(344.4) : 116.1 : 2(18.0)
CAS No. : 43229-80-7
CAT No. : KMF104045
Formula : C19H24N2O4 : 1/2(C4H4O4)
FW : 344.41 : 1/2(116.1)
K. M. Pharma Solution Pvt. Ltd., India (CIN: U72100GJ2023PTC141278), is a leading, innovation-driven company accredited by FDCA, GMP-GLP, ISO 9001:2015, and NABL (ISO/IEC 17025 & ISO 17034). Specializing in the design, synthesis, and characterization of pharmaceutical impurity standards and reference materials, we offer an extensive portfolio including nitrosamine impurities, pharmacopeial and non-pharmacopeial impurities, drug metabolites, glucuronides, process degradants, excipient-related impurities, unknown impurity isolation & structural elucidation, and stable isotope-labeled compounds.
As an integrated Contract Research, Development & Manufacturing Organization (CRDMO), we provide end-to-end scientific services — from early-stage discovery through to commercial supply — with a strong focus on quality, compliance, and customer satisfaction.
© 2026 K.M.Pharma. All Rights Reserved